

Modern science and technology usually break through in the field of interlaced superposition and gain innovation. For the original innovation, it may come from accidental discovery, or random, and the tireless hard work of scientists is the necessary premise of chance or chance. All the original innovations obtained are full of the blood and sweat of the cultivators.
Nanodiamond-carbon nano-onion and nano-polycrystalline PCD are the result of multidisciplinary cross-fusion at the nanoscale. Whether a new form of matter can be applied is mainly related to the characteristics of the substance itself. For new materials, new features are needed, and some people need to recognize and make constant attempts to find the right market to gain recognition and application.
2. Nano diamond
2.1 Preparation of nanodiamond
In 1982, the Institute of Fluid Physics and the Institute of Chemical Physics of the USSR Academy of Sciences first obtained experimental results of explosive synthesis of nanodiamonds. In 1987, Russia took the lead in reporting successful nanodiamonds. In 1988, scientists in the United States and Germany reported on the preparation technology of explosive detonation. Japan also reported the experiment of synthesizing UFD in 1989. Russia, the United States, Japan and other countries in the late 1980s have used the detonation method to synthesize nano-scale diamond ultrafine powder.
In the late 1980s, the Southwest Institute of Fluid Physics and the Northern Institute of Technology under the leadership of Prof. Yu Shouyi took the lead in launching the research on explosive synthesis of diamonds and made progress. The Lanzhou Institute of Chemical Physics and the Northern Institute of Technology of the Chinese Academy of Sciences began to study in the 1990s. Although it started late in China, it has developed rapidly and has gradually caught up with the international level. In 1993, the Lanzhou Institute of Chemicals first reported on this work.
Including the Northwest Institute of Nuclear Technology and Beijing Institute of Technology, the first research on nanodiamonds was carried out, the preparation methods and theories were perfected, and the production line was established. At present, several production lines have been built in China and a production scale of more than 100 million carats per year has been formed.
2.2 Application of nanodiamond
Some people have divided the application of nanodiamond into two parts, surface and core. It makes sense. The structure and properties of the material determine its use. Nano-diamond not only has the comprehensive characteristics of diamond, but also has good biocompatibility for the human body; it has great transmittance and absorption rate for radar waves and infrared ultraviolet light. The cold cathode field emission effect has many functional groups such as carboxyl group, hydrocarbon group and carbonyl group on the surface, which is easy to be closely combined with metal, rubber, plastic polymer and fabric surface, etc., thus providing technical basis and development space for the application of nano diamond. Therefore, the application and development of nanodiamonds should start from these two aspects.
Surface properties mainly utilize its nanoparticle properties, namely small particle size, large specific surface area, high surface energy, large proportion of surface atoms and its unique four major effects: small size effect, quantum size effect, quantum tunneling and surface. effect. Wherein, surface effects include adsorption of surface functional groups;
The performance of the core mainly utilizes the superhard performance of diamond.
Application of nanodiamond
1 for preparing a nanocrystalline sintered body;
2 additives for chemical composite plating;
3 for lubricating oils, solid lubricants and lubricating coolants;
Advantages: (1) Saving lubricant materials. (2) The friction momentum is reduced by 20% to 40%. (3) Friction surface wear is reduced by 30% to 40%. (4) Rapid running-in of the friction pair. Unit consumption of nanodiamond: 0.01 to 0.20 kg in 1 000 kg of lubricating oil;
4 possibility for infrared and microwave absorbing materials;
5 nanometer diamond for magnetic recording system;
6 for the stealth material catalysis;
7 Adding nano-diamond to rubber and polymer can improve its performance;
9 nm diamond improves fuel dispersion and combustion values ​​in fuel oil.
10 for new energy storage and energy conversion materials;
11 other potential applications
(1) Russian and British scholars have found that the nano-diamond gray powder has a dielectric constant increased by 18 orders of magnitude in a humid environment, which is the highest record of all materials including ferroelectrics. Scholars believe that it is possible that water molecules adsorb acid groups on the surface of the nanodiamond, leading to proton separation and further abrupt changes in dielectric constant.
(2) Russian researchers used a short pulse wave of carbon ions to act on silicon, and for the first time successfully studied the method of synthesizing nanodiamond particles in the surface layer of other substances. This method has a good application prospect in the semiconductor lighting industry and even the entire semiconductor industry. This synthesis method can be realized not only by silicon but also inside other substances.
(3) preparing a polymer wear-resistant coating by adding nano-diamond-carbon black-mica to polytetrafluoroethylene;
(4) Fluorescence effect and its application in medical imaging field - nitrogen-vacancy (NV) effect.
(5) Application in hot fluids. Only 0.1% concentration can increase the heat exchange efficiency by 70%, and the higher the temperature, the higher the efficiency.
3.1 Discovery of carbon nano onions
Carbon nano-onion is a member of the fullerene family. It is a three-dimensional closed structure of carbonaceous particles composed of multiple layers of concentric carbon spheres. It has a polyhedral appearance and an internal shape like onion.
Iijima S. Direct observation of the tetrahedral bonding in graphitized carbon black by high resolution electron microscopy. Cryst Growth, 1980, 50: 675-683.
Ugarte D. Curling and closure of graphific networks under electron beam irradiation. Nature, 1992, 359:707-708.
3.2 Morphology and structure of carbon nano onions
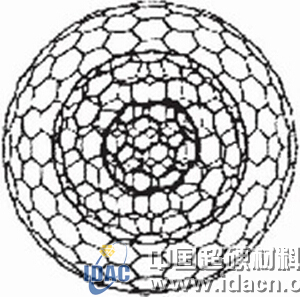
Figure 2 Carbon onion model consisting of three layers of concentric graphite layers (C60, C240, C540 from the inside to the outside)
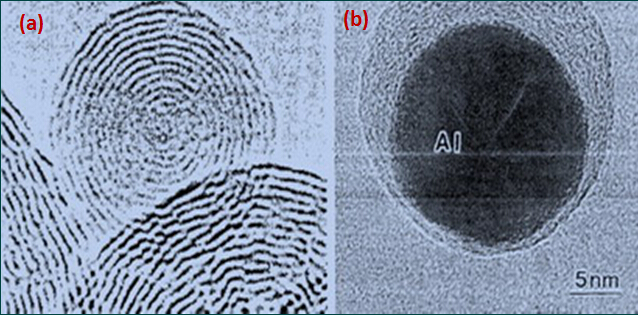
Figure 3 HRTEM image of carbon nano onion:
(a) monomeric carbon nano onions; (b) containing metal carbon nano onions
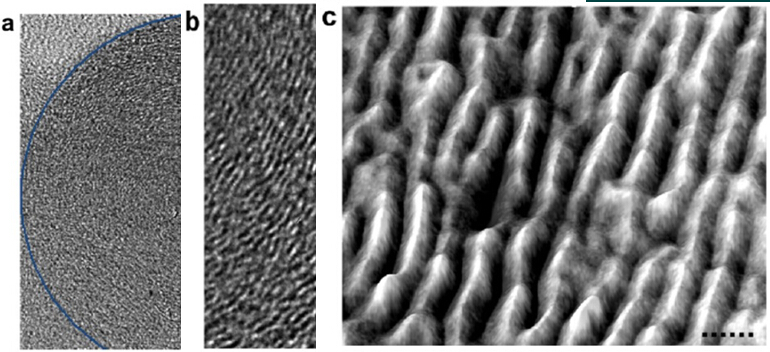
Figure 4 HRTEM of a single C-nano onion; (a) radius 25 nm layer 50; (b) individual graphite layer curved toward the nano-onion core indicated in Figure (a); (c) Figure 3b yellow frame 3D Grayscale contour projection indicates that there are significant defects in the individual graphite layers, that is, the graphite layers are interlocked and bonded to each other.
The scales of (a), (b), and (c) are 10 nm, 5 nm, and 0.5 nm, respectively.
3.3 Preparation of carbon nano onions
Since the discovery of carbon nano-onions, its unique structure and broad application prospects have attracted wide attention. Current research on such materials has focused on the exploration of a large number of preparative processes to lay the groundwork for further performance and application research.
In short, there are many methods for preparing carbon nano onions, and various types of raw materials are also various. In theory, all carbonaceous materials can be converted into carbon nano-onions by certain preparation methods. However, there are still many problems in the preparation of carbon nano-onions, such as low product purity, many structural defects, low yield, high preparation cost, etc., which is not conducive to further research on its performance and application.
If a suitable raw material and a process for improving the treatment can be found, it is expected that the macro-preparation of the carbon nano-onion can be realized by this simple process. The C-nano onion obtained by the vacuum heat treatment method using the nano-diamond produced by the detonation method has less impurities, the process is simple, and the sample yield is not limited by equipment, and is suitable for macro production.
The project team systematically studied the method of preparing C-nano onion by vacuum annealing using nano-diamond prepared by detonation method, and discussed the process and formation mechanism of nano-diamond conversion to C-nano onion in the literature. The structural characteristics of this method and C-nano onion prepared from the raw materials were discussed.
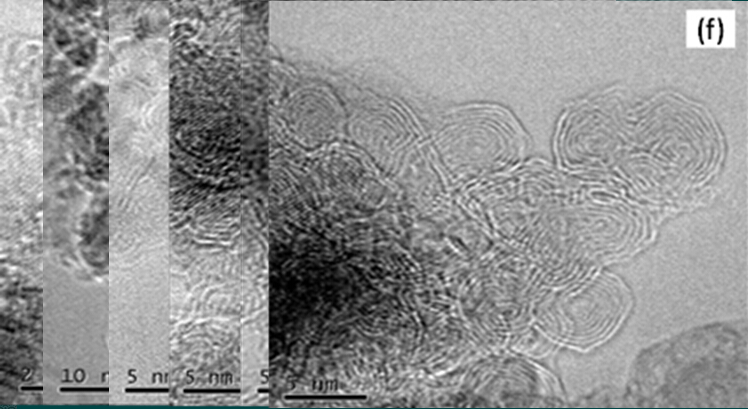
Figure 5 HRTEM image of annealed samples of nanodiamond at different temperatures:
(a) 500 °C; (b) 800 °C; (c) 900 °C; (d) 1000 °C; (e) 1100 °C; (f) 1400 °C
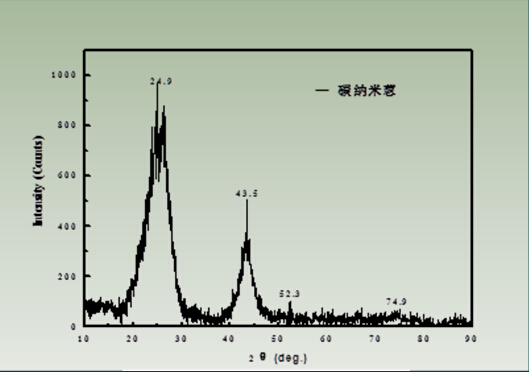
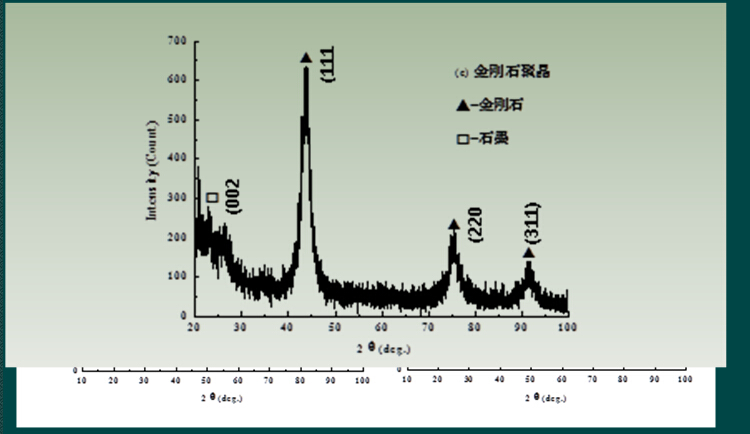
Figure 6 X-ray diffraction pattern of carbon nano onion
3.4 Application of carbon nano onions
In the 1980s, Kroto et al. discovered C60, one of the major scientific discoveries of the 20th century. The C60 research advanced at an unprecedented rate. Since 1999, people began to pay attention to the performance of carbon nano-onion, mainly for the preparation and performance of a small number of shells, such as two layers (composed of C60 and C240) and three layers (composed of C60, C240 ​​and C540). test.
Due to the nano-particles of carbon nano-onion, special surface layer structure and material properties, it has a wide application space.
1 Superconducting applications. C60 has a hollow structure that, if filled with certain special metal nanoparticles, can have many unique properties. First, filling a suitable metal atom can greatly change the conductivity of fullerenes, and it is expected to make a high conductor or even make it a superconductor;
2 For lubricants and rubber boosters. The organic compound or metal particles are surrounded by the outer graphite layer and have good corrosion resistance and are not affected by oxidation or decomposition. Hirata et al. tested the friction properties of carbon nano-onions obtained by heat-treating diamond clusters and particles with a disk plate composed of silicon wafers and steel balls, indicating that the carbon nano-onion has high compression resistance and a small friction coefficient, which can be used as Lubricants, rubber enhancers, etc.
3 medical radiotracer, contrast agent and radiopharmaceutical transport carrier. The metal-coated carbon onion is expected to be used as a radiotracer and a radiopharmaceutical due to its spherical structure, high stability, and low toxicity to tissue cells. For example, graphite-coated radioactive metal-coated carbon nano-onions can bring metal atoms into the body for radiological diagnosis and tracing purposes, especially as a new material for contrast agents.
4 used in the field of photovoltaic and fuel cell production. Kamat et al. found in the study of carbon nanotubes that the size of the specific surface area is more important for higher catalytic activity for methanol oxidation and oxygen reduction. It is concluded that carbon nano-scallions with higher surface area than single-walled carbon nanotubes may also have a great impact on the current miniaturization of fuel cells.
5 used as a chemically stable reaction cluster and a special catalyst.
The film prepared by the 6 carbon nano-onion has nonlinear optical properties and can be used as a photoelectronic material and a magnetic data recording film material.
7 Carbon nano onions also have certain potential uses in gas storage.
8 is used for the preparation of a nanocrystalline polycrystalline sintered body.
9 is used for the preparation of graphene. Nanoscale graphene was prepared by the MA method.
Fullerene has only been discovered for more than 20 years, and the performance and application of fullerenes have been slow in the early stages of discovery. Fullerene, a spherical molecule, has received a lot of attention from scientists in all fields of the world. The main reason is that it has too much potential to be developed and utilized. With the deepening and development of carbon nano-onion research, the understanding of its structure and performance will become more and more profound and comprehensive. Carbon nano-scallions will surely be obtained in many aspects of people's daily life and in many other important fields. widely used.
4. Nano polycrystalline diamond sintered body
4.1 Sintered body using pure graphite carbon as precursor
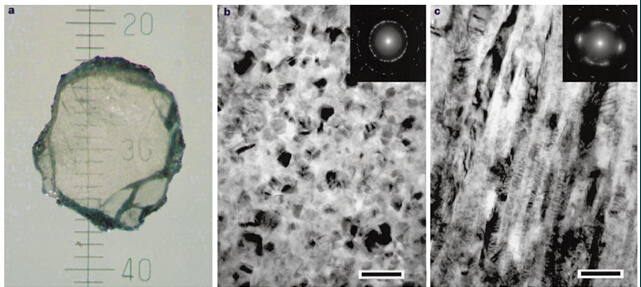
Irifune, T. et al. used pure graphite (99.9995%) in 2003 to prepare a nano-polycrystalline diamond sintered body (Gr) without binder at ultrahigh pressure (12-25GPa) high temperature (2300-2500 °C). Grain size 10-20nm, hardness 110-130GPa
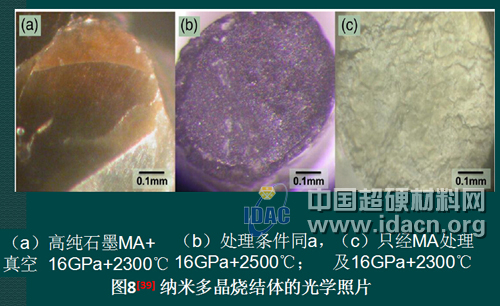
4.2 Sintered body using non-graphite carbon as precursor
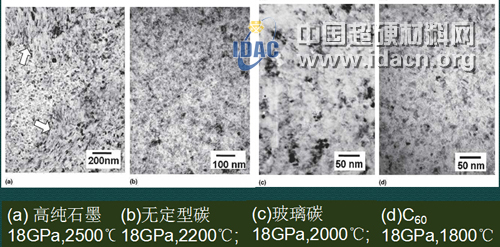
Figure 9 [38] TEM photo of nanocrystalline polycrystalline diamond sintered body directly transformed by high temperature and high pressure of different raw materials
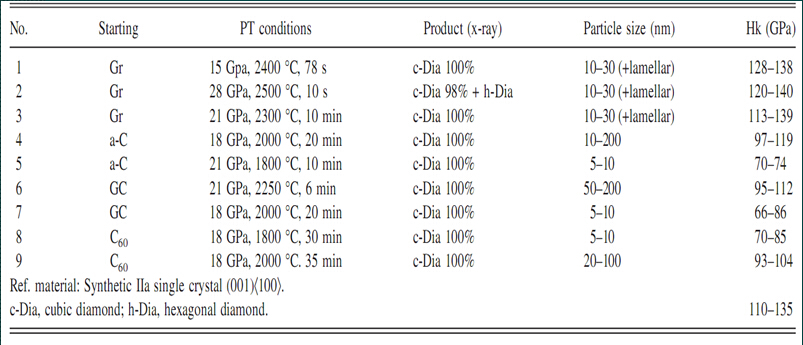
Table 1 [38] Structure, grain size and hardness of different raw materials obtained under different process parameters
The nano-diamond synthesized by detonation method not only has the general characteristics of diamond, but also has the small size effect of nano material and the great specific surface area, especially containing more dislocations and lattice distortion, which makes it have high sintering activity. In theory, it is easier to achieve sintering under relatively low pressure and temperature conditions, and is therefore considered to be an ideal raw material for the manufacture of nano-PCD materials.
But also because nanodiamond has a large specific surface area, has a strong surface activity, makes it adsorb a large number of impurity atoms and groups, and is prone to agglomeration, losing some of its excellent special properties as nanomaterials, hindering Its application in high-pressure sintering of nano-diamond bulk materials. Therefore, research on key technologies such as nano-diamond purification technology, surface purification technology, and surface graphitization, plastic deformation, nano-polycrystal formation and recrystallized grain growth control in high-pressure sintering, and then find the ideal sintering process. The research focus of relevant science and technology workers at this stage.
The project team uses the nanodiamond size effect (2-12nm) and surface effect, and has many characteristics of dangling bonds. After removing the adsorbate by vacuum treatment, chemical substitution, self-adsorption agglomeration and other techniques, the project attempts to achieve no or micro-assisted sintering. synthesis. Figure 10-13 shows the XRD pattern of nano-diamonds treated by various atmospheres. Except for the argon atmosphere, different atmospheres can purify the nano-diamonds to a certain extent and keep the diamond structure unchanged.
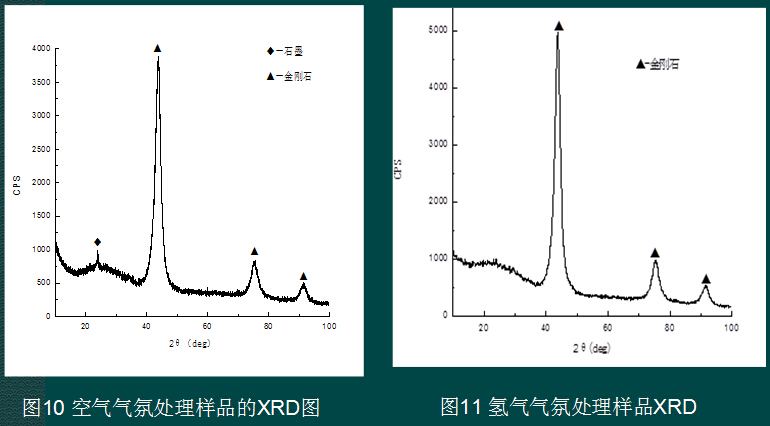
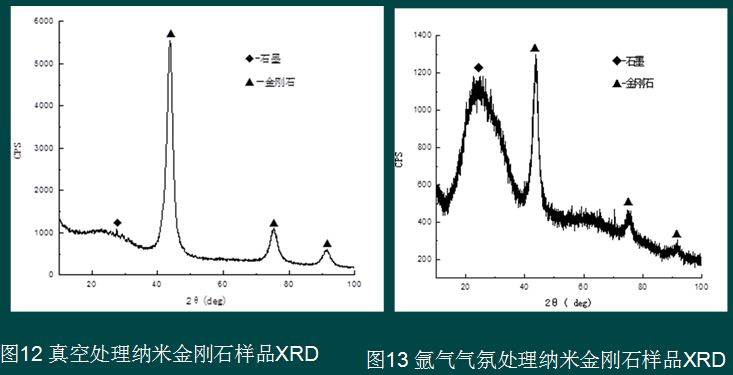
4.4 Sintered body prepared from carbon nano onion
1 Preparation under industrial high pressure conditions
Using C-nano onions as precursors 1100-1350°C+4.5-6.5GPa
The nanocrystalline polycrystalline sintered body has a grain size of 10-30 nm
Hardness Hv 44GPa
A sample of micron-sized diamond particles was added to the C-nano onion precursor. The nanocrystalline and micron-diamond interfaces were well bonded, with no obvious interface and hardness of 71 GPa.
It is convenient to prepare nanocrystalline polycrystalline sintered body on ordinary domestic six-sided top press

On the basis of this, Gong Wen focused on the effects of nano-onions prepared under different temperature conditions on the properties of nanocrystalline polycrystalline sintered bodies. The conclusion is that under the high temperature (4.5-6.5GPa) high temperature conditions commonly used in industrial production, it contains inclusions. The diamond core C-nano onion is more easily converted into a nanocrystalline sintered body;
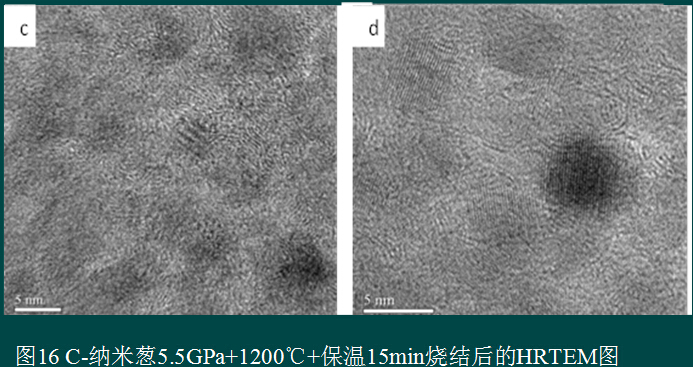
Yu Qianghua studied the microstructure of nanocrystalline polycrystalline sintered bodies under different process conditions, and improved the pressure transmission attenuation during sample preparation by adding micron-sized diamond particles, so that the hardness of the sample reached 71GPa.

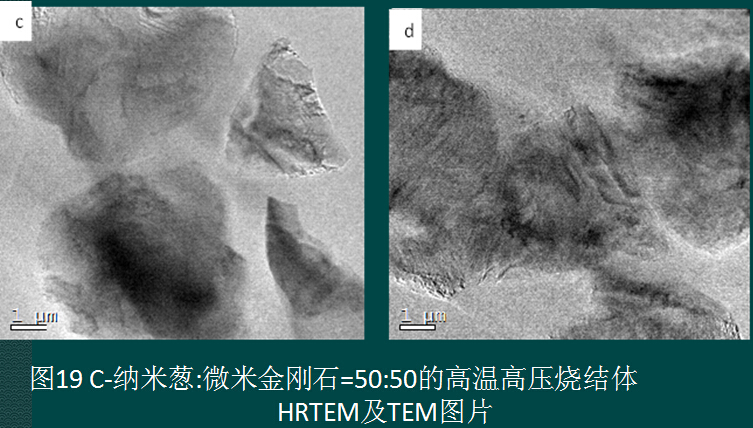
2 Preparation under ultra-high pressure conditions
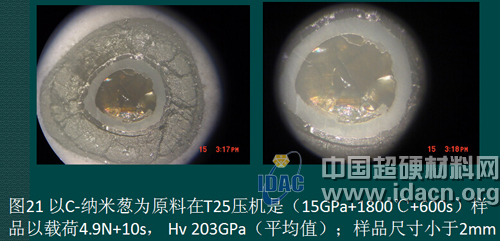
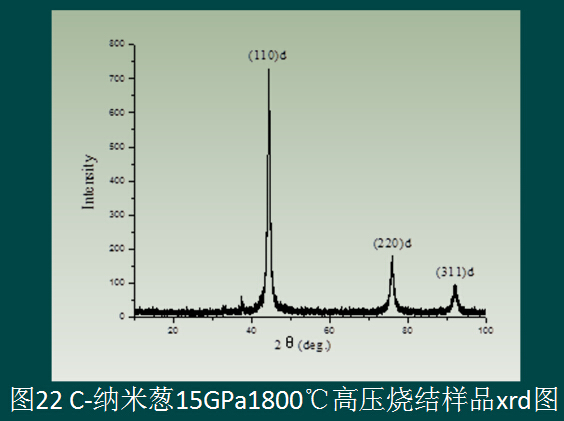
The self-made C-nano onion is also used as the precursor. On the T25 press, the appearance of the sample is 15GPa+1800°C+600s. The appearance of the sample is shown in Figure 20. If it can be further studied, it should be nearly transparent. Crystal diamond sintered body. The hardness was measured on a KB5 BVZ microhardness tester to obtain an average hardness of Hv203GPa.
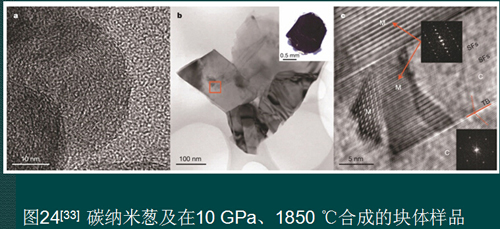
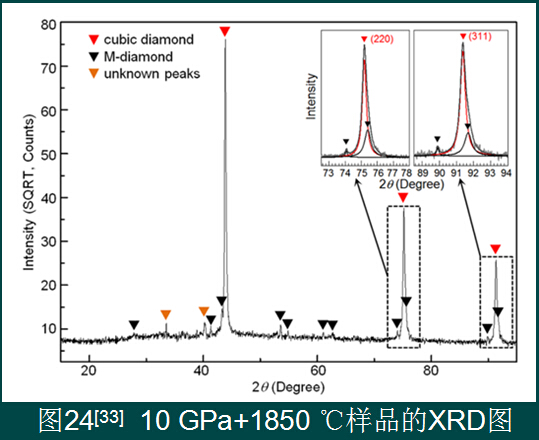
5. Prospects for the preparation of nanocrystalline polycrystalline sintered bodies without additives
In the research history of non-bonded nano-polycrystalline sintered bodies, whether using high-purity graphite, amorphous carbon, glassy carbon or C60, in addition to this group, all use ultra-high pressure (15GPa or more) + ultra-high temperature (above 1800 °C), although the performance is good, the high pressure and temperature are difficult to achieve in the industrial equipment, and it is difficult to solve in the short term. Moreover, the size of the obtained sintered body is also limited; the manufacturing cost is also unusually high, and practical applications encounter challenges.
The C-nano onion is a direct conversion of precursors into nanocrystalline polycrystalline diamond sintered bodies, realizing the actual production and application of such sintered bodies. Now we have the industrial preparation method of C-nano onion. The raw material supply is sufficient, and the equipment needed is the existing production equipment. Although there are still problems such as low hardness and incomplete conversion, the crux of the problem has been found, and the problem is solved. Time problem.
Compared with other precursors, C-nano onion has a special structure, and its C-shell shell electron has a mixed structure of sp2 and sp3, which reduces the phase transition activation energy to diamond transformation, and because of this multilayer structure The layer has more defects and also provides a growth point that combines two adjacent C-nano onions to transform the diamond.

Nano-diamond-carbon nano-onion-nano-polycrystalline sintered body is a special kind in nano-materials. It is an extreme type in scale and hardness, and it is a wonderful work in the exploration of human science and technology. Special performance gives it a special application, which requires people to dig constantly and will benefit humanity. (This article was translated from the PPT of the speech of the second executive director (expansion) meeting of the 5th China Superhard Materials Association, without the author's own review)
Ball lenses are a completely spherical lenses most commonly used for improving signal coupling between fibers, emitters and detectors. Aside from fibre coupling applications, ball lenses are also used as objective lenses in endoscopy, laser measurement systems and bar-code scanning. Ball lenses are manufactured from a single substrate of glass and can focus or collimate light, depending upon the geometry of the input source.
Ball lenses are great optical components for improving signal coupling between
fibers, emitters, and detectors. They are also used in endoscopy, bar
code scanning, ball pre-forms for aspheric lenses, and sensor
applications. Ball lensesare
manufactured from a single substrate of glass and can focus or
collimate light, depending upon the geometry of the input source.
Half-ball lenses are also common and can be interchanged with full ball
lenses if the physical constraints of an application require a more
compact design.
CCRQ can supply Sapphire
Ball Lens,UV Fused Silica Ball Lens,Ruby Ball Lens,k9 Glass Ball
Lens,Znse Ball Lens, ZnS Ball Lens,Silicion Ball Lens and Half Ball lens
with material K9,JGS1,Sapphire, Znse, ZnS, Silicon etc


K9 Glass Ball/Half Ball Lens,Sapphire Ball/Half Ball Lens,Ruby ball lens, Fused SIlica Ball Lens,Silicon Ball/Half Ball Lens, Znse Ball/Half Ball Lens,Germanium Ball lens, CaF2 Ball Lens
Changchun Ruiqi Optoelectronics Co.,Ltd , https://www.ruiqi-optics.com