[ Huaqiang Security Network News ] 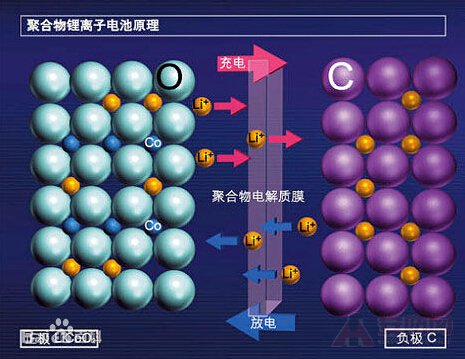
This article introduces the working principle of lithium-ion battery. First of all, we introduce the concept of lithium-ion battery. Lithium-ion battery is a secondary battery (rechargeable battery), which mainly relies on lithium ions between the positive and negative electrodes. Move to work. During charging and discharging, Li+ intercalates and deintercalates between the two electrodes: when charging, Li+ is deintercalated from the positive electrode, and the electrolyte is embedded in the negative electrode, and the negative electrode is in a lithium-rich state; The battery is generally made of a material containing lithium as an electrode, and is a representative of modern high-performance batteries.
Lithium batteries are classified into lithium batteries and lithium ion batteries. Both mobile phones and laptops use lithium-ion batteries, which are commonly referred to as lithium batteries. True lithium batteries are rarely used in everyday electronics because of their high risk.
Principle of lithium ion battery
Lithium-ion batteries use a carbon material as the negative electrode and a lithium-containing compound as the positive electrode. No lithium metal exists, only lithium ions. This is a lithium ion battery. A lithium ion battery is a general term for a battery in which a lithium ion intercalation compound is used as a positive electrode material. The charging and discharging process of a lithium ion battery is a process of intercalating and deintercalating lithium ions. In the process of intercalation and deintercalation of lithium ions, it is accompanied by embedding and deintercalation of equivalent electrons with lithium ions (commonly, the positive electrode is represented by embedding or deintercalation, and the negative electrode is represented by insertion or deintercalation). During charge and discharge, lithium ions are intercalated/deintercalated and inserted/deintercalated between the positive and negative electrodes, and are aptly referred to as "rocking chair batteries".

When the battery is charged, lithium ions are generated on the positive electrode of the battery, and the generated lithium ions move to the negative electrode through the electrolyte. The carbon as the negative electrode has a layered structure, and it has many micropores. The lithium ions reaching the negative electrode are embedded in the micropores of the carbon layer, and the more lithium ions are embedded, the higher the charging capacity. Similarly, when the battery is discharged (ie, the process we use the battery), the lithium ions embedded in the carbon layer of the negative electrode come out and move back to the positive electrode. The more lithium ions return to the positive electrode, the higher the discharge capacity.
Generally, the charging current of the lithium battery is set between 0.2C and 1C. The larger the current, the faster the charging and the greater the heat of the battery. Moreover, excessive current charging, the capacity is not full, because the electrochemical reaction inside the battery takes time. Just like pouring beer, if it is too fast, it will produce bubbles, but it will not be full.
Full House Solution,Hanging Cabinet For Small Bedroom,Cream Bedside Cabinets,Floating Bedside Cabinet
Ningbo Oulin Import&Export Co.,Ltd. , https://www.oulin-oversea.com